Добро пожаловать на сайт, который поможет Вам научиться готовить простые, сложные, алкогольные и очень полезные напитки дома, а также вкусные закуски и десерты к ним.

Закуски
Научитесь готовить бутерброды, сандвичи и бургеры на любой вкус в домашних условиях для закуски и перекуса между основными приемами пищи. Узнайте, как правильно транспортировать и хранить горячие и холодные закуски для сохранения качеств блюда и его вкуса.

Напитки
Узнайте, как правильно готовить вино в домашних условиях из различных ингредиентов. Научитесь смешивать лучшие коктейли, варить компоты и настаивать лимонады. Изучите все правила приготовления и правильной подачи чая согласно любым мировым традициям.

Десерты
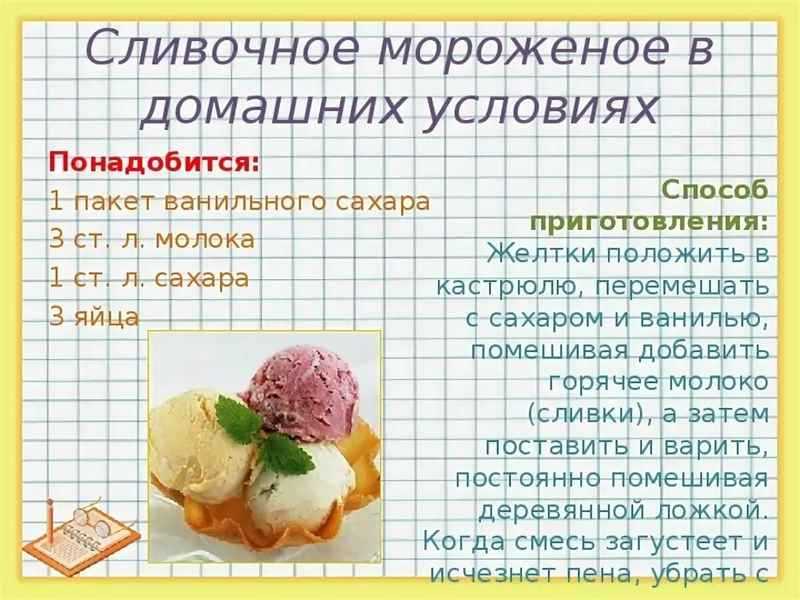
Научитесь готовить самые разнообразные десерты и правильно их подавать с соответствующими напитками: рецепты, фото, видео, советы по подаче от профессионалов кулинарного дела.
Описание сайта
Здесь Вы найдете на сотнях страниц полезной информации именно то, что ищете для создания идеального напитка или закуски, десерта к нему. Лучшие рецепты оригинальных и традиционных вариантов приготовления, все возможные виды домашних алкогольных напитков от самогона до вина, пива или настоек. Узнайте, как правильно подавать аперитив и нужна ли к нему закуска. Что нужно предлагать гостям для поддержания легкой заинтересованности к основной трапезе и как закончить застолье легким десертом.